The History of Batteries: From a Sulphur Ball to Lithium Cells
History
August 17. 2022
5 min.
A battery is made up of multiple electrochemical cells, where each of them has a positive electrode (cathode) and a negative electrode (anode) in contact with an electrolyte. A battery stores chemical energy, and converts it into electrical energy. The chemical reactions in a battery involve the flow of electrons between the electrodes through an external circuit. The history of batteries began almost 400 years ago. To better understand their development, it is useful to realise that the primary purpose of this invention was to generate and store electrical energy.
The Experiments That Led to the Modern Battery
One of the first attempts at generating electricity in the modern era was creating a static charge. In 1660, the German physicist Otto von Guericke created an electrical machine by using a large ball of sulphur that, when spun and rubbed, could attract feathers or small pieces of paper and generated electric sparks.
In 1744, the German physicist Ewald Georg von Kleist constructed the first Leyden jar. A Leyden jar is a glass container filled with water, into which a metal wire is inserted through a cork. One electrode is the water, the other the hand holding the jar. The glass acted as a dielectric. While the Leyden jar resembles a battery, it was in fact the world’s first capacitor.
One of the first steps towards the discovery of an electrochemical cell was taken by the Italian naturalist and physician Luigi Galvani. In 1791, when dissecting frogs at the University of Bologna, he noticed that when he touched frog legs with metal items, their muscles twitched. This phenomenon became known as 'animal electricity”. Galvani’s observation was probably the first discovery of the principle behind batteries.
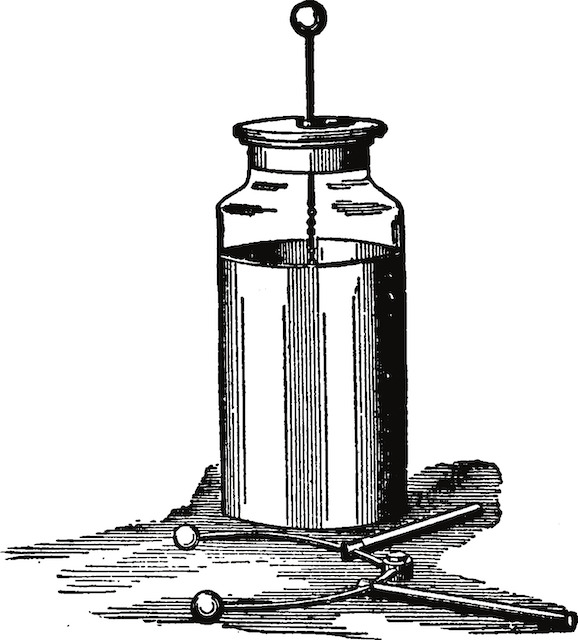
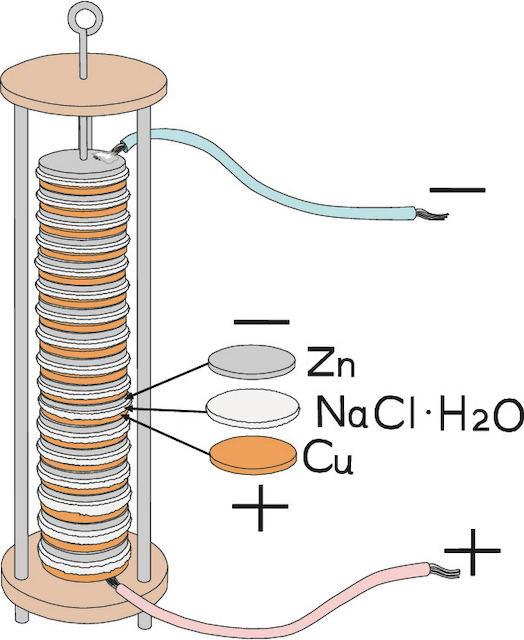
The mechanism was later explained by another Italian, Alessandro Volta, who devised many experiments. He found out, for example, that certain liquids combined with pairs of different metals can generate a constant stream of electrical energy. In the case of the frogs, the liquid was stored in the cells. Based on this discovery, Volta started to experiment with various combinations of metals – zinc, lead, tin and iron as the positive plate, and copper, silver, gold and graphite as the negative plate. The results of his experiments were published in 1800. The very first electric cell, known as the voltaic cell, publishedinvented by Volta, consisted of a copper and zinc electrode in a solution of sulphuric acid. Volta also found that he cancould increase the voltage by combining several cells together into a voltaic pile.
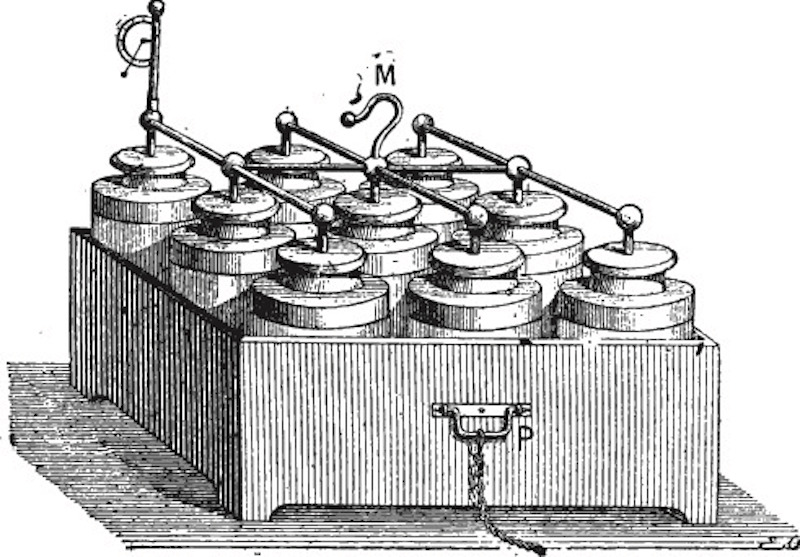
In 1802, the Scottish chemist William Cruickshank constructed the first electrical battery that could be mass produced. It was a closed rectangular container with an arrangement of equally sized square plates of copper and zinc inside. The container was filled with brine or a diluted acid to act as the electrolyte. The resulting battery was similar to those we use today.
In 1836, the English chemist John F. Daniell developed an improved battery that provided a more stable current than the Volta device. Originally, batteries were single-use and could not be recharged. But in 1859, the French physicist Gaston Planté improved the concept and proposed a rechargeable cell. The system was based on lead and an acid, which is still used to this day.
The First Alkaline Batteries
In 1899, the Swedish scientist Waldmar Jungner patented a rechargeable nickel-cadmium cell with electrodes made of these two metals in a solution of potassium hydroxide. This was the very first battery with an alkaline electrolyte. At this time, the only competitor was the lead battery, which is both physically and chemically less resistant. Junger also attempted to replace cadmium with various amounts of iron, but the results were not satisfactory.
It took two more years for the American inventor Thomas Alva Edison to successfully replace cadmium with iron and construct a working nickel-iron battery which he patented in 1902. It consisted of a positive nickel electrode, negative iron electrode, and potassium hydroxide as electrolyte. The cell’s disadvantages, however, were the low specific energy, weak performance at low temperatures and poor charge retention. Despite that, Edison’s Ni-Fe battery was widely used, for example, as a backup source for railroad crossing signalisation or in lamps used in mines.
The term 'battery' was coined by Benjamin Franklin, the American naturalist, inventor and politician, in 1749. He used it to describe a series of connected capacitors that he studied in his electricity experiments. The capacitors were glass plates coated with metal. In the experiments, the capacitors were charged by a static generator and discharged by touching a metal to the electrode. Linking them together into a battery enabled a stronger discharge. Originally, the term 'battery' simply meant two or more similar objects working in tandem. Later it was applied to voltaic piles and similar devices where multiple electrochemical cells were combined, in a way similar to Franklin’s capacitors. Today, the term is also commonly (but inaccurately) used for single electrochemical cells.
At that point, the best solution for portable devices was still the nickel-cadmium battery. In Sweden, Ni-Cd batteries were launched on the market in 1910, but they only reached the USA in 1946. The first models were robust and had a much higher energy density than the commonly used lead cells. But they were also more expensive.
In 1932, two German scientists, Leo Schlecht and Hartmut Ackermann, improved the longevity of Ni-Cd cells by inventing sintered plates. (Sintering is a process in which nickel powder is melted under high pressure and at a temperature far lower than its melting point). This improved load currents and extended the battery’s lifespan. A major development occurred in 1947 with a technology that allowed recombining gases created during charging. This enabled the development of fully hermetically sealed Ni-Cd cells.
These Ni-Cd batteries remained the most commonly used power source for decades, even though today, there is no alternative that would achieve the same combination of lifespan, reliability and temperature range as the Ni-Cd cell.

In 1991, Sony launched the first lithium-ion battery on the market, and in 1997 the lithium-polymer battery. In lithium-polymer batteries, the electrolyte is a solid polymer composite instead of a liquid solvent, and the electrodes and separators are laminated. Because of that, the battery can be sealed in a flexible container and shaped to fit a specific device. This together with their small size is the main reason that lithium cells are now used in most small portable devices.

Over the course of several centuries, battery research progressed from the early experiments on a sulphur ball and frog legs to many different technologies of batteries that we know today. They all have different energy density and longevity, maintenance requirements, and other properties that need to be considered when selecting a suitable battery for our purposes.
Podobné články
The First Rechargeable. A brief History of Lead-acid Batteries
December 12. 2024
3 min.
VíceComing Back Stronger. The History of GAZ part IV
November 21. 2024
2 min.
VícePowering the Workers' Paradise. History of GAZ part III
July 11. 2024
2 min.
VícePowering Innovation with Clay and Animal Parts
May 21. 2024
2 min.
Více



