The First Rechargeable. A brief History of Lead-acid Batteries
History
December 12. 2024
3 min.
Although one and a half centuries old, lead-based batteries are not only still widely used, but even keep on evolving.
In 1801, only two years after the invention of the Voltaic pile, the French scientist Nicolas Gautherot observed during his electrolysis experiments that platinum or silver wires would provide a brief current after being detached from the pile. This gave a certain Gaston Planté the idea that if a reverse current would be passed through the apparatus, the pile could be recharged, and the first accumulator was born. Or that's how the story goes according to Planté's groundbreaking Recherches sur l' Electricité published in 1879.
In fact, similar observations as Gautherot's were made in 1803 by the German physicist Johann W. Ritter using electrodes made of gold wire. Based on this, he devised a pile which allowed to use these 'secondary currents' to recharge it to some extent. He experimented with piles consisting of layered discs of copper, platinum, silver or iron, and only with lead, he could not obtain the desired outcome. So, in a way, it's Ritter's contrivance that could be regarded as the first accumulator. However, at the given time, his invention fell more or less into oblivion.
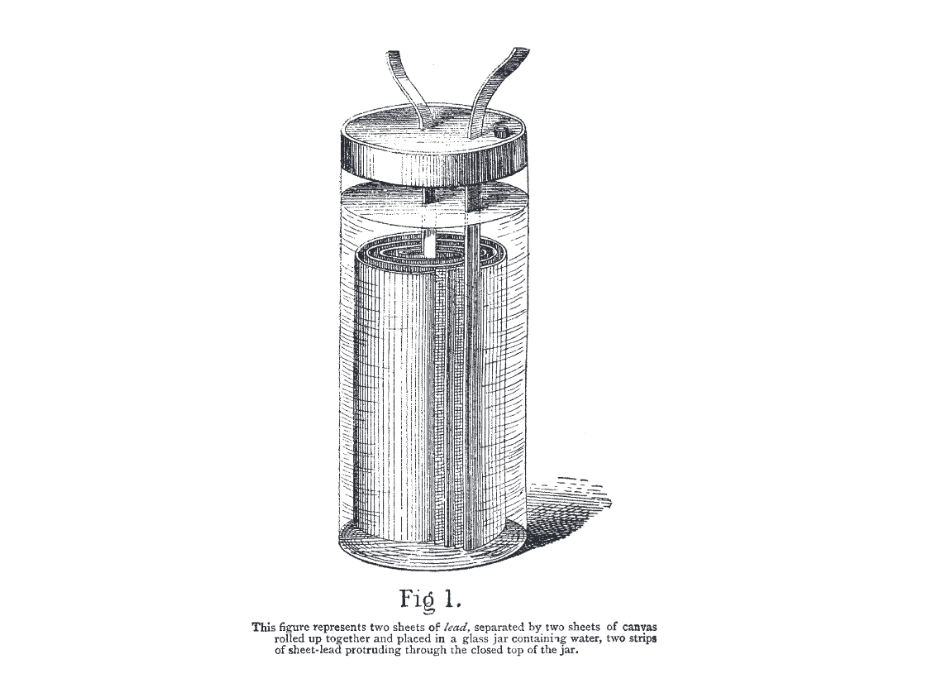
In 1854, the German physician and physicist Wilhelm J. Sinsteden succeeded in replicating the aforementioned effect by using two large lead plates in a vessel of diluted sulfuric acid. But the most functional design was indeed the 'pile secondaire' presented by Planté in 1859. It consisted of two spiral-wound sheets of lead immersed in a glass jar filled with a solution containing about 10 percent sulfuric acid. They were initially separated by caoutchouc strips, but the rubber did not last very long and was later replaced by a thick cloth.
Refining the concept
The electrodes were initially made of pure lead, and it took a whole day and three Daniell cells to oxidize the surface of the positive plate, or to charge the battery. Planté also found that after the first charge, its capacity was noticeably lower than later on. It was only after several cycles that the lead on the cathode became spongy while the surface of the anode was overgrown with porous peroxidized lead, or PbO2, increasing the electrodes' surface. Which is how the need for battery formation was discovered.
The forming helped, sure. But it took – now brace yourself – three months of repeated charging to produce enough crystalline PbO2 on the positive plate and to stabilize a film of porous lead on the opposite plate. That was until 1880, when the French chemical engineer Camille A. Faure reduced forming time to some 60 hours by giving the plates a preliminary coating of red lead (Pb3O4) which significantly sped up the reduction of lead, or the oxidation to lead peroxide respectively, as well as increased the cell's capacity.
Shortly thereafter, the English metallurgist John Scudamore Sellon improved on Faure's battery by placing the red lead paste into a perforated plate to improve adhesion, and by using lead-antimony alloys as electrode material instead of pure lead. The 1880s saw even more improvements in electrode design when a ribbed structure was patented by Charles F. Brush while S. C. Currie invented the basic form of tubular plates still used for traction and stationary lead-acid batteries.
Still keeping the pace
More substantial improvements to these designs came only about half a century later, in the 1930s. Alloy grids based on antimony were surpassed by lead-calcium and lead-selenium alloys, or at least in batteries used for infrequent cycling applications. This, together with an improved grid structure, made the electrodes more rigid, allowing them to be thicker and carry more active material. About the same time, lead-acid batteries began using silica gel instead of liquid electrolyte, making them practical for portable devices and in other applications where electrolyte leaking had been an issue.
However, it took another quarter of a century until batteries were able to operate in truly any position. Invented by Otto Jache in the US, but commercially available only since the 1970s, the sealed or valve-regulated lead-acid (VRLA) battery is more practical in other respects, too. Overcharging a lead-acid battery leads to water being decomposed into hydrogen and oxygen. But from a VRLA, the gas can't escape and recombines within the battery, making frequent refilling a thing of the past.
The red lead paste has, of course, also been steadily refined. In modern batteries, it contains carbon black, barium sulfate, and either lignosulfonate or sulfonated naphthalene condensate in order to improve both cycling and battery life. During the 20th century alone, the energy density of lead-acid batteries thus increased many times while the water loss issue was solved thanks to VRLA. And while environmental concerns over lead usage persist, innovations such as carbon-enhanced electrodes and hybrid lead-carbon batteries seem promising.
So, new and fancy battery technologies may come and go, but lead-acid batteries are to stay, at least for the foreseeable future. Similarly to nickel-cadmium batteries, they provide a highly reliable and cost-effective energy storage solution for many crucial applications.
| Born 1834 in Orthez in southwestern France, Gaston Planté also contributed to our understanding of static electricity as opposed to currents, explored electric phenomena in the atmosphere, and even conceived applications of electricity in the fields of dentistry and surgery. And in 1855, as 'a studious young man full of zeal,' Planté discovered the first fossils of the two-meter-tall flightless bird Gastornis parisiensis. Yes, he named the animal after himself. But most importantly, Planté never protected his inventions by patents, enabling their quick adoption. He still made a fortune but used it to convert his house in Bellevue near Paris into a retirement home for impoverished scientists. He also funded the establishment of a biannual monetary prize in the field of electricity to be awarded by the Académie des Sciences. |
If you want to learn more about the dawn of the lead-acid battery and Planté himself, we highly recommend the following article:
Kurzweil, P. (2010). Gaston Planté and his invention of the lead-acid battery — The genesis of the first practical rechargeable battery. Journal of Power Sources, 195(14), 4424–4434. doi: 10.1016/j.jpowsour.2009.12.126
Related articles
Powering Innovation from a Clay Jar
May 21. 2024
2 min.
MoreOver a Century Old, But Still Modern
December 23. 2022
4 min.
MoreThe History of Batteries: From a Sulphur Ball to Lithium Cells
August 17. 2022
5 min.
More



