Powering Innovation from a Clay Jar
History
May 21. 2024
2 min.
The first truly useful battery was certainly a far cry from what we consider a battery today, but at its time, the Daniell cell represented a major technological breakthrough.
In 1780, Luigi Galvani discovered what he called 'animal electricity' by bringing in contact two metals and touching two different parts of a frog's muscle, causing it to contract. Some twenty years later, his compatriot Alessandro Volta invented the voltaic pile, a stack of galvanic cells consisting of two metal discs submerged in brine. Fast forward to the 1830s, voltaic piles still were the only means of generating electricity. Thereby they enabled a series of major scientific breakthroughs, but the practicality of voltaic pile was more than questionable as the cell voltage dropped rapidly during discharge. In other words, it couldn't power anything in any useful way.
Until the British chemist and meteorologist John Frederic Daniell set about to improve the voltaic cell so that it would draw power more evenly and who knows, maybe one day, allow for some practical applications of this new and fascinating phenomenon called electricity. While Volta himself did not consider the reaction in his apparatus to be of chemical nature at all, Daniell and his contemporaries began to realize that electricity is not an inherent quality of certain metals, but the outcome of interactions between different materials.
And while Volta did not regard electrolyte to be of significant importance within his cell, Daniell found out that the energy loss in voltaic cells was due to the water in the electrolyte getting involved in the reaction, leading to a buildup of hydrogen bubbles at the copper electrodes. So, he introduced a second electrolyte to consume the hydrogen produced by the copper(II) sulfate solution into which he submerged the cathode.
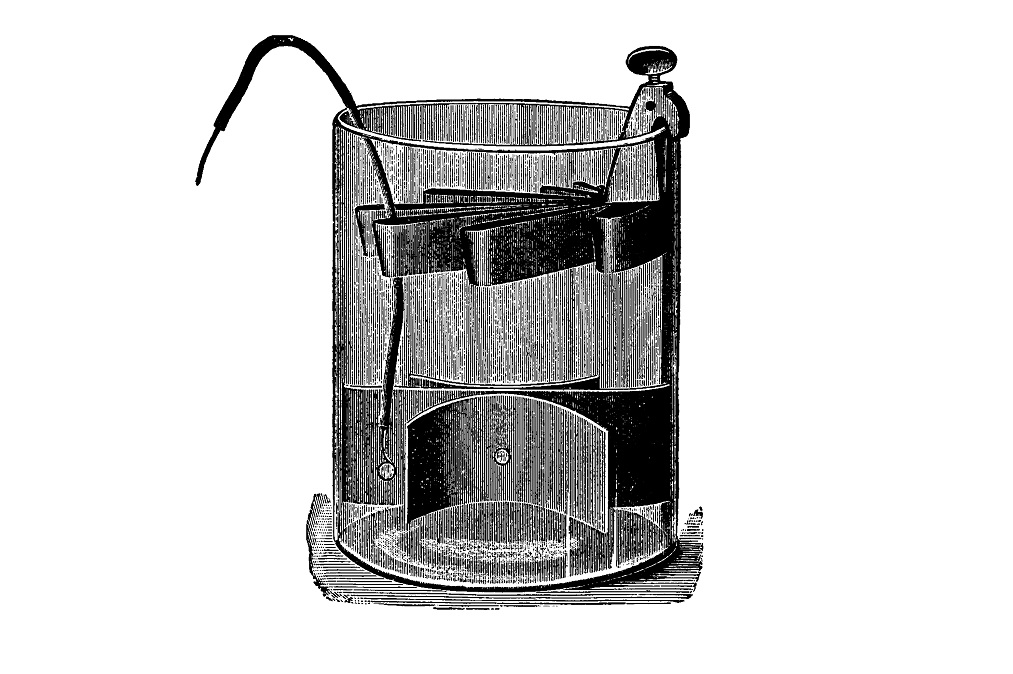
But because the reactions on both electrodes are interdependent, the two half cells must be connected in a way that allows ions to flow freely between them. There are several ways to do this; for instance, your chemistry teacher may have demonstrated the Daniell cell with two separate glass containers, one for each electrode, connected by a so-called salt bridge. But Daniell's original design consisted of a copper cylinder filled with a copper sulfate solution, into which a tube of ox gullet was suspended containing a sulfuric acid solution. The passage of ions was enabled by the ox gullet acting as a porous membrane.
Some two decades later, sulfuric acid was replaced by zinc sulfate by a certain J. F. Fuller. And sometime in the 1860s, a French inventor by the name Callaud simplified Daniell's setup by just placing the copper electrode in the bottom of an earthenware jar and pouring over the copper sulfate solution. Above it came a less concentrated zinc sulfate solution, and because ZnSO4 is less dense, it floats on the copper sulfate without mixing. This arrangement meant that the battery could only be used in stationary applications, otherwise the solutions would mix. And spill, because the jar had no lid.
However, also called gravity or crowfoot cell, Callaud's design was less costly to produce than Daniell's arrangement while yielding a stronger current. It quickly became the battery of choice for telegraphy, especially in Britain and America. But, as it is often the case in times of technological transformation, it was equally quickly surpassed by a more advanced concept. In this case, by the Leclanché cell which used ammonium chloride as electrolyte, a carbon cathode and zinc on the anode. Still, the Daniell cell was the first battery that was of any meaningful use in practice, for which it and its inventor should always be remembered.
| What is a volt? As per definition proposed at the 1881 International Conference of Electricians, a volt is the electromotive force of a Daniell cell. Although according to current definitions, the standard potential of the Daniell cell at room temperature is actually 1.1 V. |
Similar articles
The First Rechargeable. A brief History of Lead-acid Batteries
December 12. 2024
3 min.
ViewFrom Now on Electric. The History of GAZ part II
March 29. 2024
2 min.
ViewThe History of Batteries: From a Sulphur Ball to Lithium Cells
August 17. 2022
5 min.
View



